STEM for Kids: How to Make a Coin Battery
This post may contain affiliate links.
Did you know that you can make a homemade battery with coins? This coin battery will actually produce a voltage similar to a small battery! This coin battery makes about 1 volt of power.
How to Make a Coin Battery
Supplies you will need to make this coin or penny battery:
6-8 Copper pennies or other copper coins (or more)
6-8 Nickels (or more) – Washers or aluminum foil could also work here.
Paper towels or Coffee Filters
1/4 c. White vinegar
1 Tbsp. Salt
Multimeter, also called a voltmeter (voltage testing meter)
Small LED Pin Light

Instructions:
In a small bowl, mix together the salt in the vinegar until the salt is dissolved.
Cut the paper towel (or coffee filters) into small squares. You will want them smaller than the coins so they are not overlapping.

Dip the squares into the vinegar and salt mixture and layer the coins in a pattern: penny, paper, nickel. You will want to finish with a penny on one end and a nickel on the other end.
Once you have a large stack, you can test it out with your multimeter. You will want to turn it to a low voltage setting and test it out. To light up an LED light bulb, you will need to reach about a 2 V reading on the multimeter. Ours didn’t quite make it that high. Ours read just under 1 V.
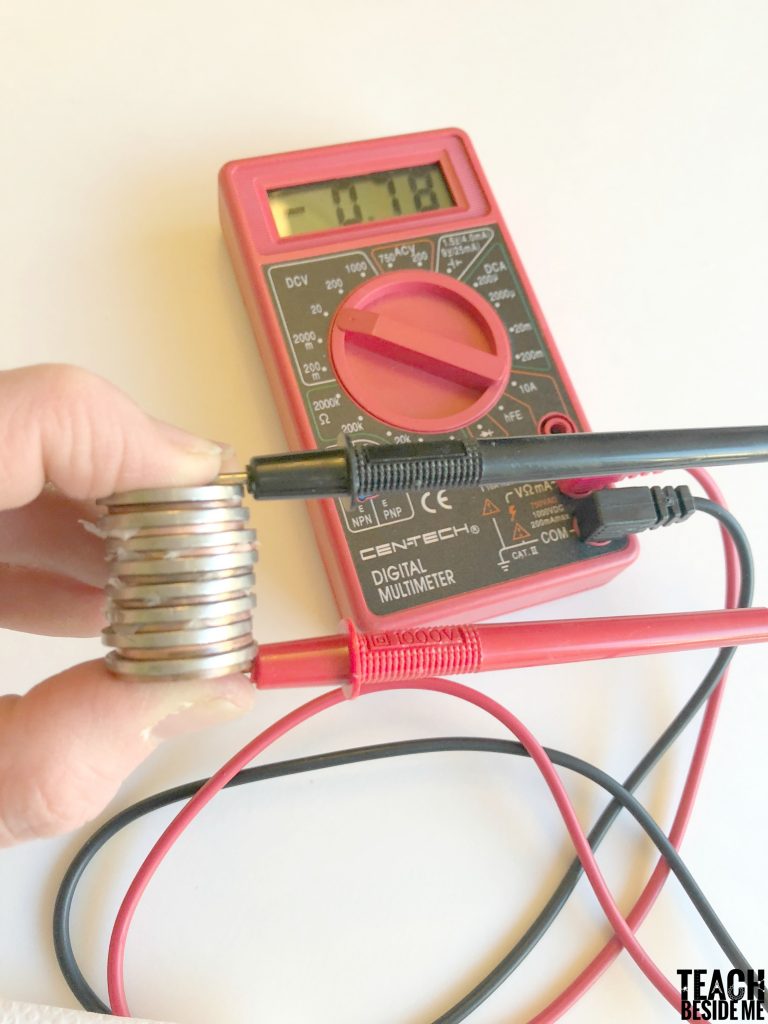
Troubleshooting: Switch the leads on the multimeter to see if you get a better reading. Another issue is having too much liquid. Wipe off excess drips to get a better reading. See if you can generate enough power to light an LED light! My son kept building ours higher to see how high the reading would go. Test other ideas to get a higher reading.
How Does the Coin Battery Work?
This is a replica of the first electrical battery made by an Italian chemist named Alessandro Volta and is also called a voltaic pile. He created an electrical current or an electrochemical reaction with the first battery using copper and zinc discs separated by felt or cardboard disks soaked in salt water (an electrolyte).
Nickels are made of zinc and pennies are made of copper. They are both conductors of electricity. When two different metals are connected by an electrolyte (which is the vinegar and salt solution), a chemical reaction called oxidation occurs at the surface of the metals. These metals are the electrodes. When these electrodes are connected by a wire, they can create an electrical current.
This post is part of the 28 Days of STEAM Series over at Left Brain Craft Brain. This week’s topic is Technology. Hop over to her site to see more awesome STEAM ideas for teaching.

Want More STEM Technology Project Ideas?
Check out these ones:
Dirt Battery
Lemon Battery
Play Dough Circuits
Conductivity Experiment






